
File2713 pH Scale01.jpg
Download this stock image: Diagram of the pH scale with examples of acidic, neutral and alkaline substances. - G156N5 from Alamy's library of millions of high resolution stock photos, illustrations and vectors.

Diagram of the pH scale with examples of acidic, neutral and alkaline substances Stock Photo Alamy
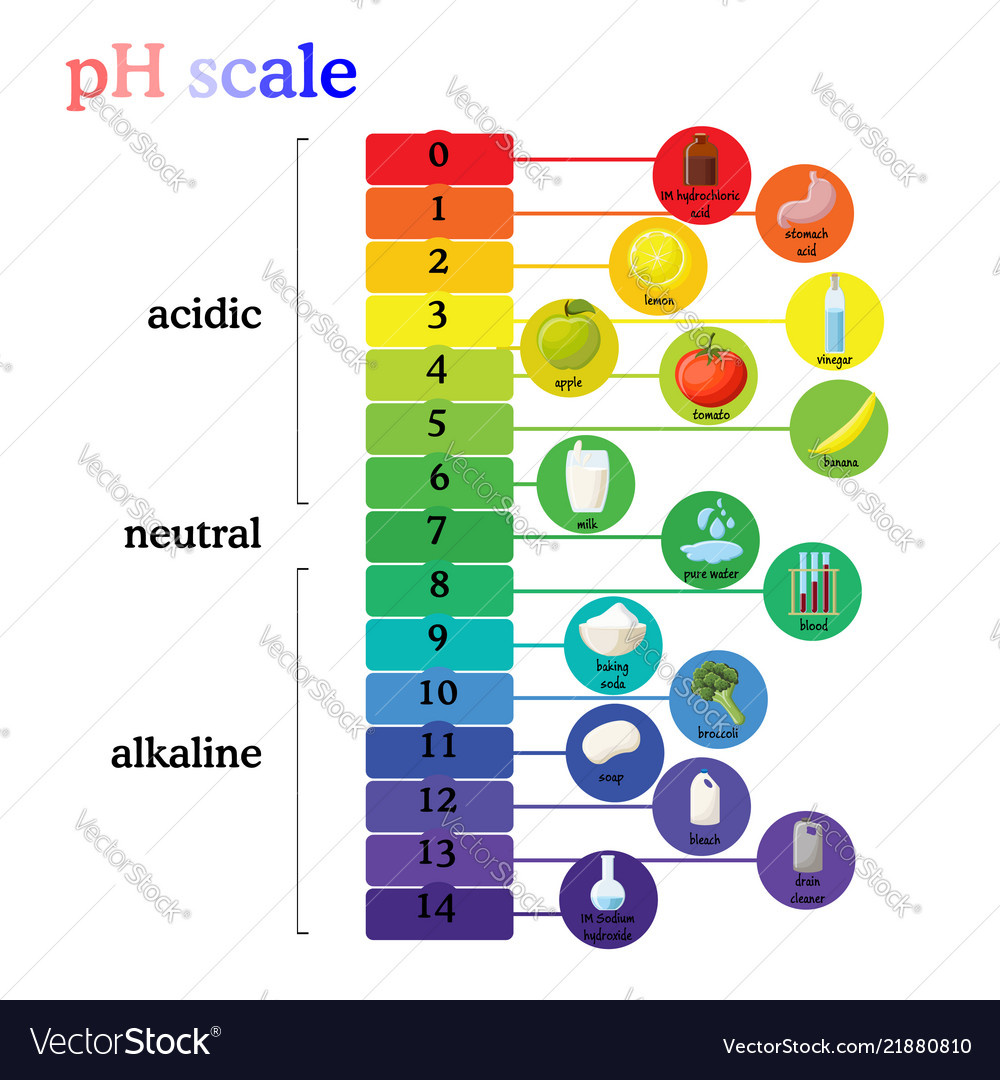
The pH scale is a commonly used scale to measure the acidity or the basicity of a substance. The possible values on the pH scale range from 0 to 14. Acidic substances have pH values ranging from 1 to 7 (1 being the most acidic point on the pH scale), and alkaline or basic substances have pH values ranging from 7 to 14.
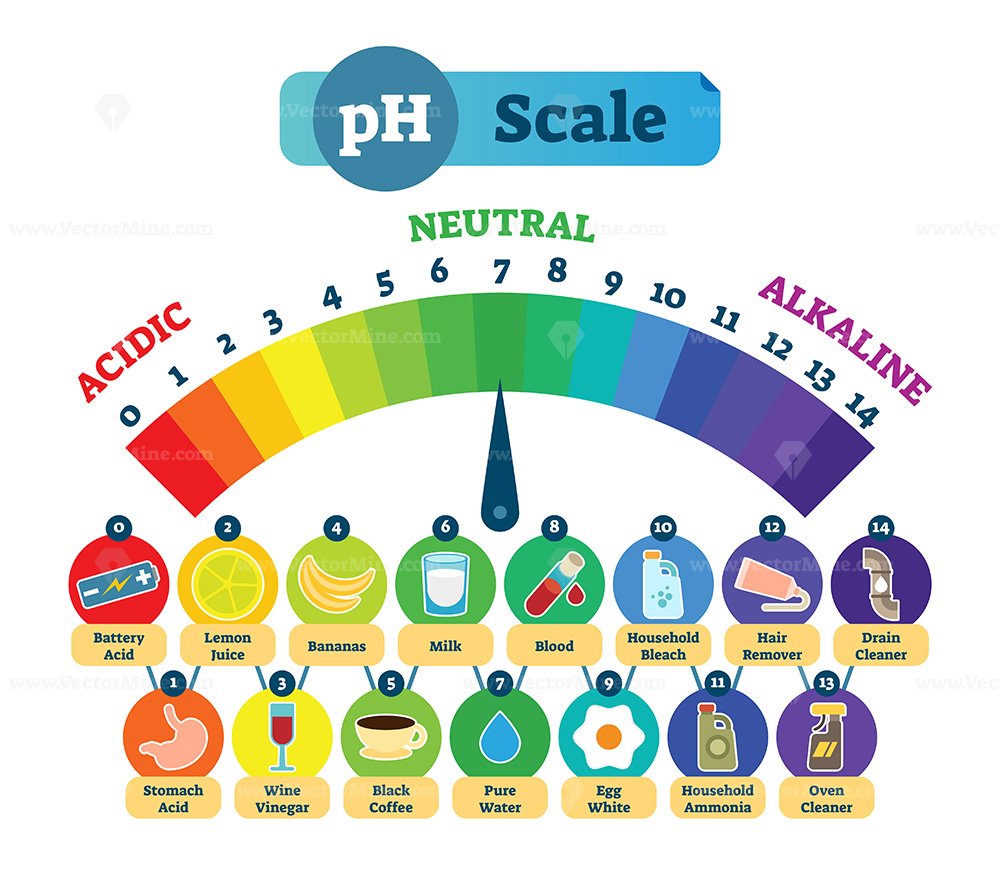
PH Acid Scale Vector Illustration Diagram with Acidic, Neutral and Alkaline examples VectorMine
The pH scale is the range of value s from 0 to 14 that describes the acidity or basicity of a solution. You can use \(pH\) to make a quick determination whether a given aqueous solution is acidic, basic, or neutral.. The diagram below shows all of the interrelationships between [H3O+][H3O+], [OH−][OH−], pH, and pOH.

The ph scale diagram 541433 Vector Art at Vecteezy
pH Scale - PhET Interactive Simulations
What’s The Reaction Between An Acid And A Base?
To give you the short answer: An acidic solution has a high concentration of hydrogen ions (H + ), greater than that of pure water. A basic solution has a low H + concentration, less than that of pure water. To see where this definition comes from, let's look at the acid-base properties of water itself. Autoionization of water
The ph scale diagram 589313 Vector Art at Vecteezy
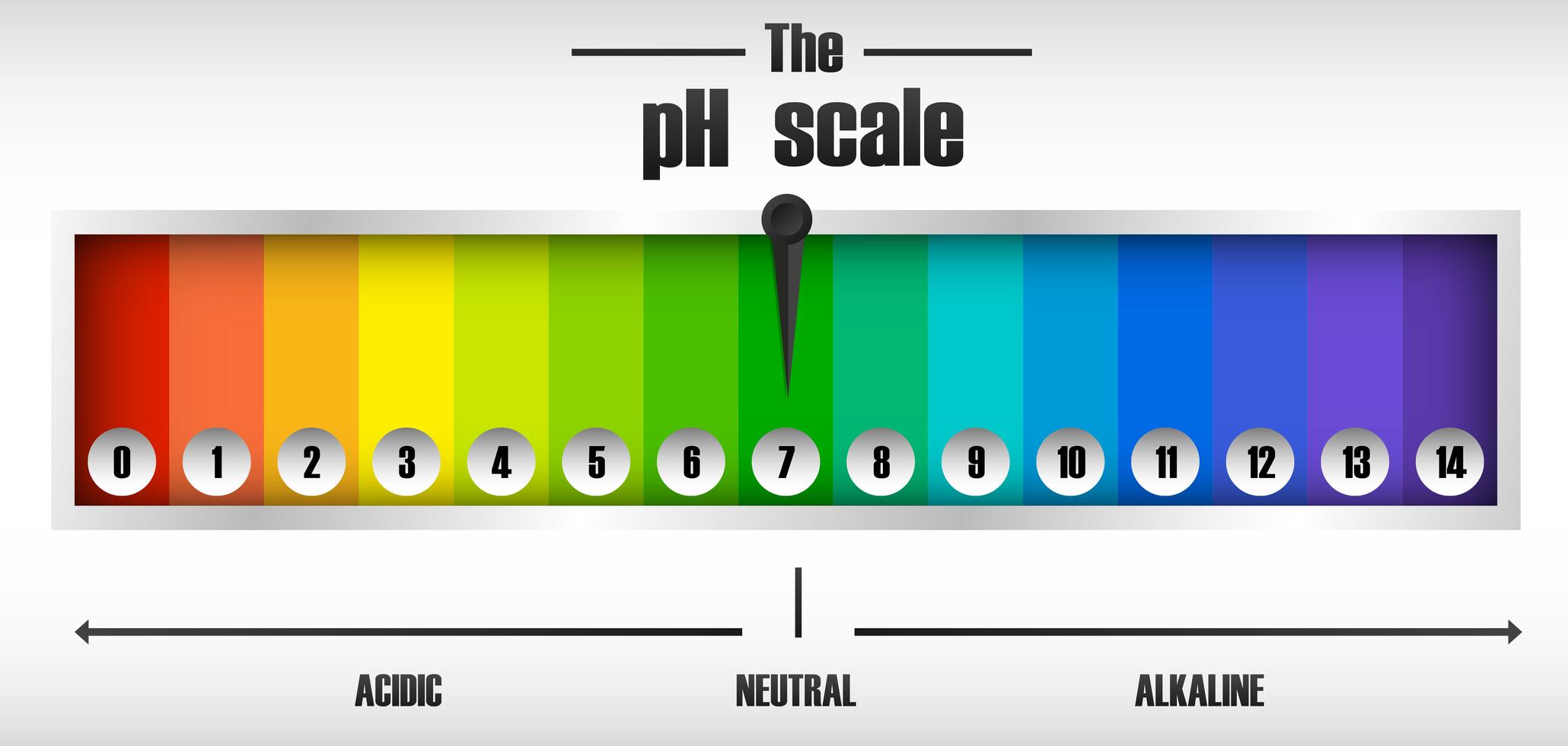
Solution. The neat and labeled diagram of the pH scale is as shown. The range of pH is from 0 to 14. pH = 7 corresponds to neutral pH. pH less than 7 corresponds to acidic pH. pH more than 7 corresponds to alkaline pH. Was this answer helpful?

pH Scale newagenutrients
In chemistry, pH (/ p iː ˈ eɪ tʃ / pee-AYCH), also referred to as acidity or basicity, historically denotes "potential of hydrogen" (or "power of hydrogen"). It is a scale used to specify the acidity or basicity of an aqueous solution.Acidic solutions (solutions with higher concentrations of hydrogen (H +) ions) are measured to have lower pH values than basic or alkaline solutions.
Ph scale diagram with corresponding acidic Vector Image
The pH scale is a number scale from 0 to 14. It tells us how acidic or alkaline an aqueous solution is. The pH scale is used to classify solutions as acidic, alkaline or neutral. Neutral.

Back to Basics Acids, Bases & the pH Scale Precision Laboratories
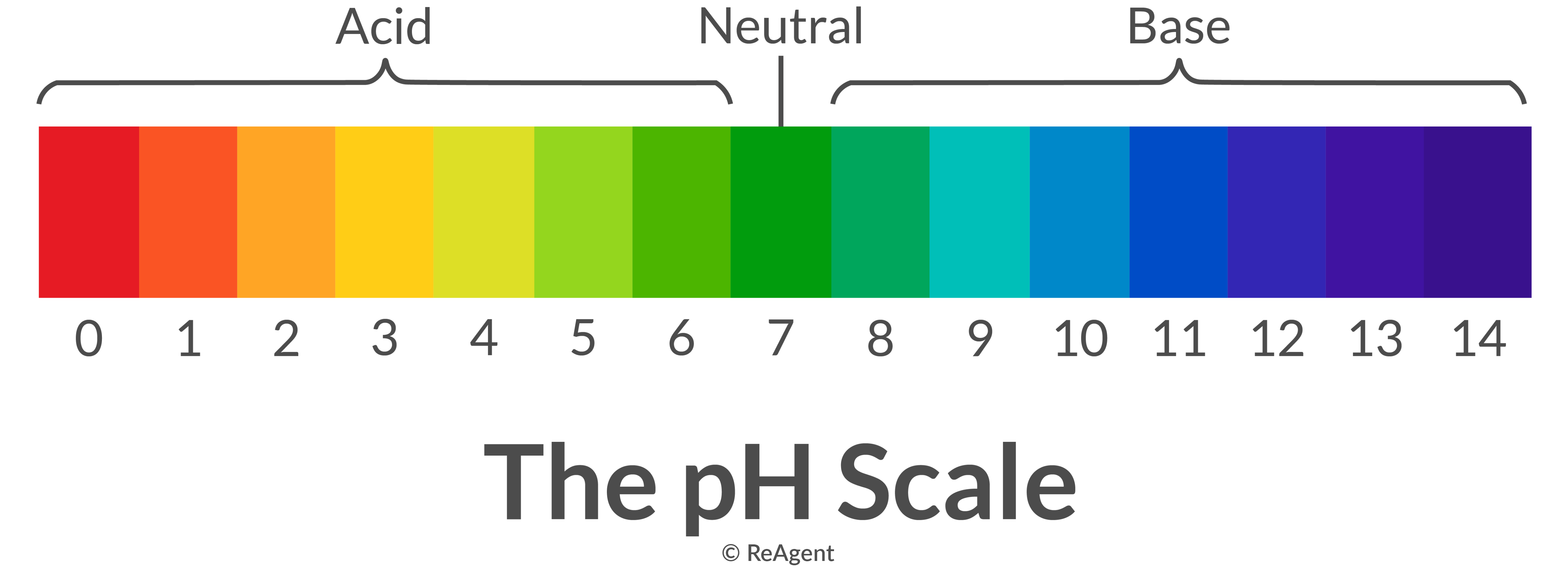
The pH scale shows how acidic or basic a chemical is in aqueous solution (mixed with water). The scale runs from 0 (most acidic) to 14 (most alkaline or basic), where 7 is neutral pH. Chemicals with pH values from 0 up to 7 are acids, those with a pH value of 7 are neutral, and those with pH values greater than 7 up to 14 are bases.
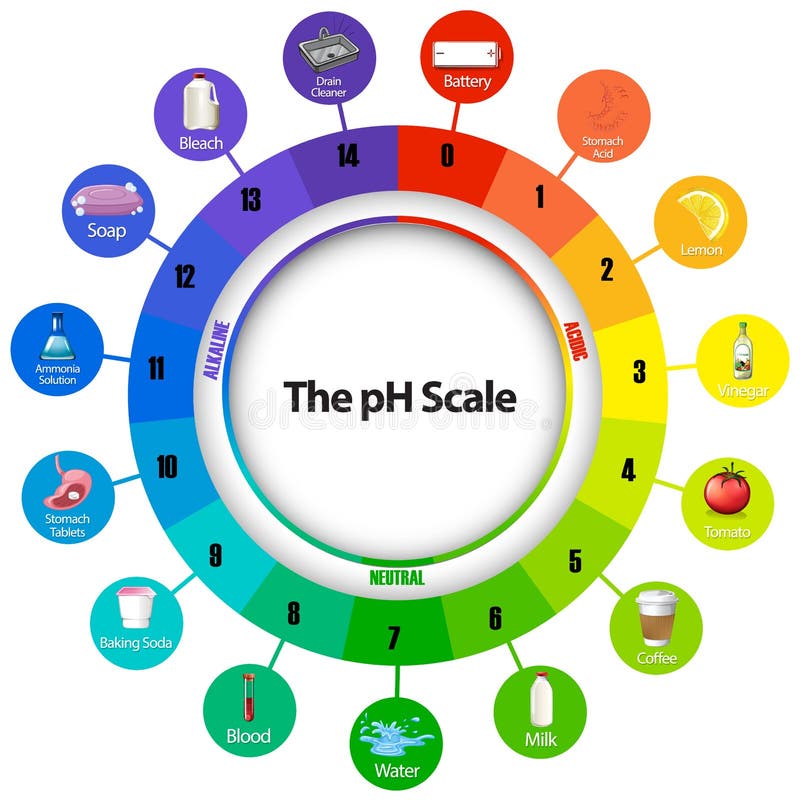
Diagram showing ph scale stock vector. Illustration of science 165346456
The range of pH goes from 0 to 14. A value less than 7 indicates that water is acidic. A value greater than 7 indicates that water is alkaline. A value equal to 7 shows that water is neutral. Each whole pH value below 7 is ten times more acidic than the next higher value. For example, a pH of 5 is ten times more acidic than 6.
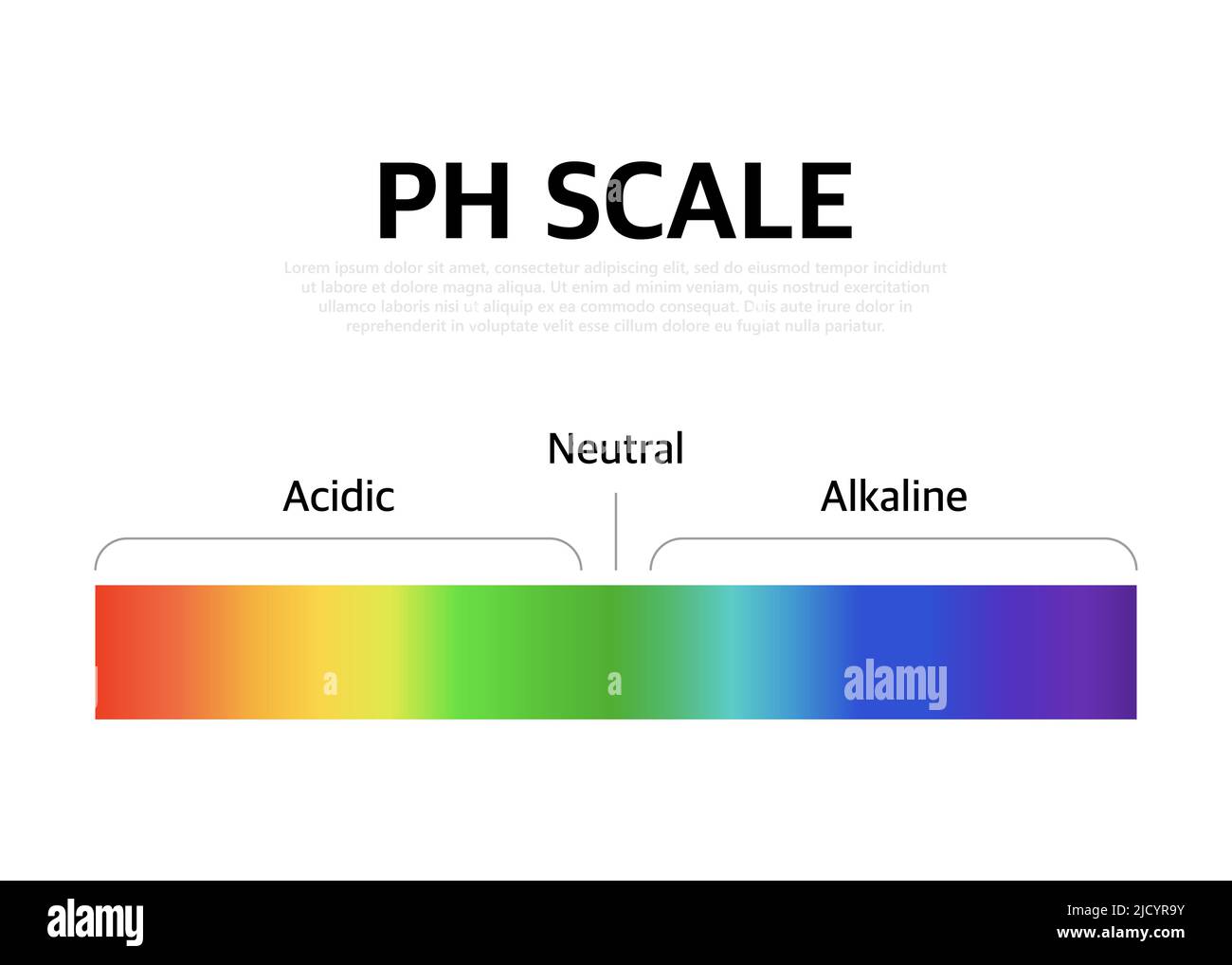
The Ph scale universal Indicator ph Color Chart diagram. Vector illustration with ph scale Stock
Eh-pH diagram, any of a class of diagrams that illustrate the fields of stability of mineral or chemical species in terms of the activity of hydrogen ions (pH) and the activity of electrons (Eh).

How Learning the pH Scale Can Create a More Balanced Diet Natural Bio Health
Draw neat and labeled diagram of pH scale? Medium Solution Verified by Toppr The neat and labeled diagram of pH scale is as shown. The range of pH is from 0 to 14. pH 7 corresponds to neutral pH. pH less than 7 corresponds to acidic pH. pH more than 7 corresponds to alkaline pH. Was this answer helpful? 0 0 Similar questions

pH strip and pH scale. Download Scientific Diagram
Google Classroom Definitions of pH, pOH, and the pH scale. Calculating the pH of a strong acid or base solution. The relationship between acid strength and the pH of a solution. Key points We can convert between [ H +] and pH using the following equations: pH = − log [ H +] [ H +] = 10 − pH We can convert between [ OH −] and pOH
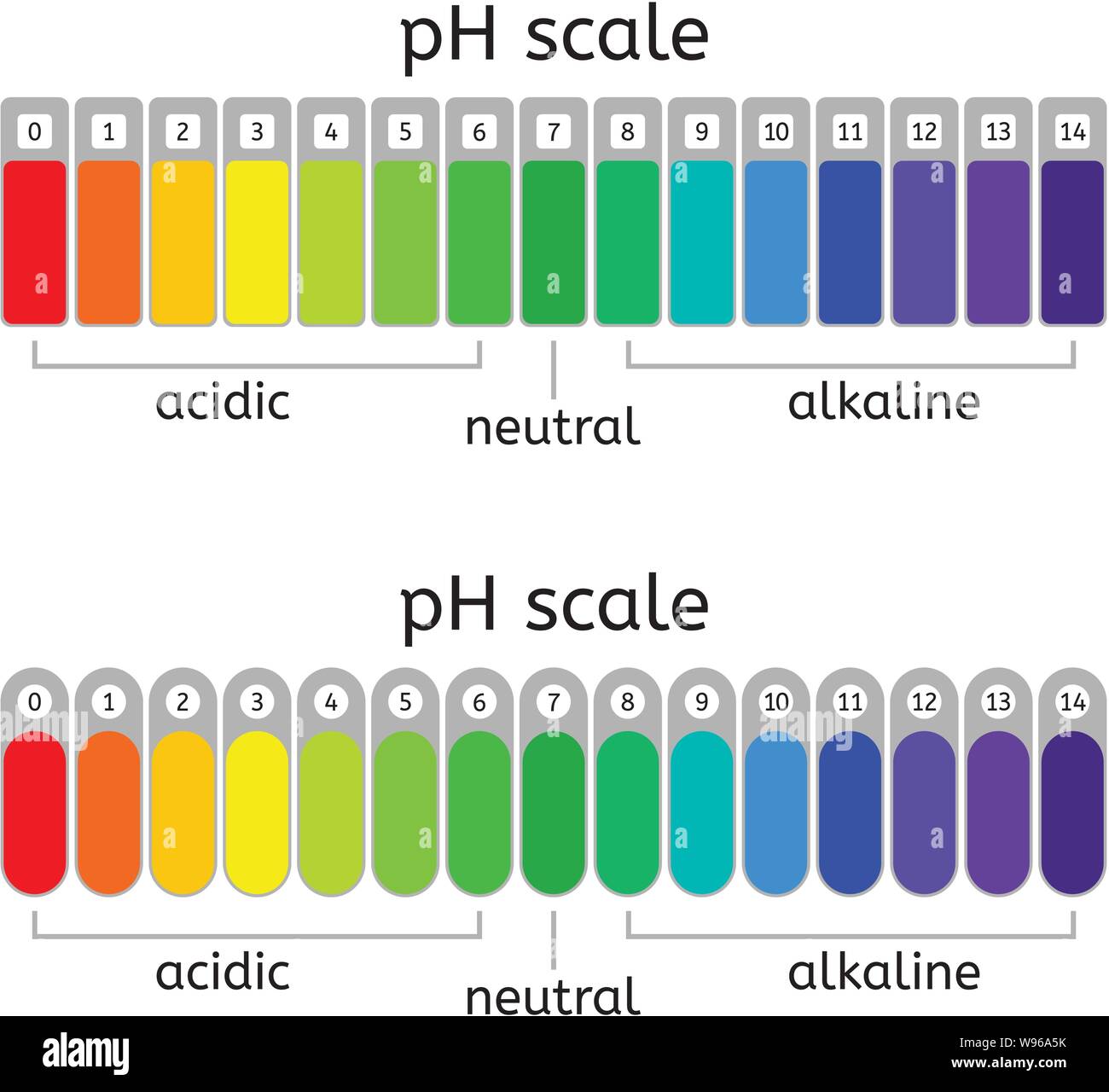
vector ph scale of acidic,neutral and alkaline value chart for acid and alkaline solutions. ph
The pH scale is the range of value s from 0 to 14 that describes the acidity or basicity of a solution. You can use \(pH\) to make a quick determination whether a given aqueous solution is acidic, basic, or neutral.. The diagram below shows all of the interrelationships between [H3O+][H3O+], [OH−][OH−], pH, and pOH.
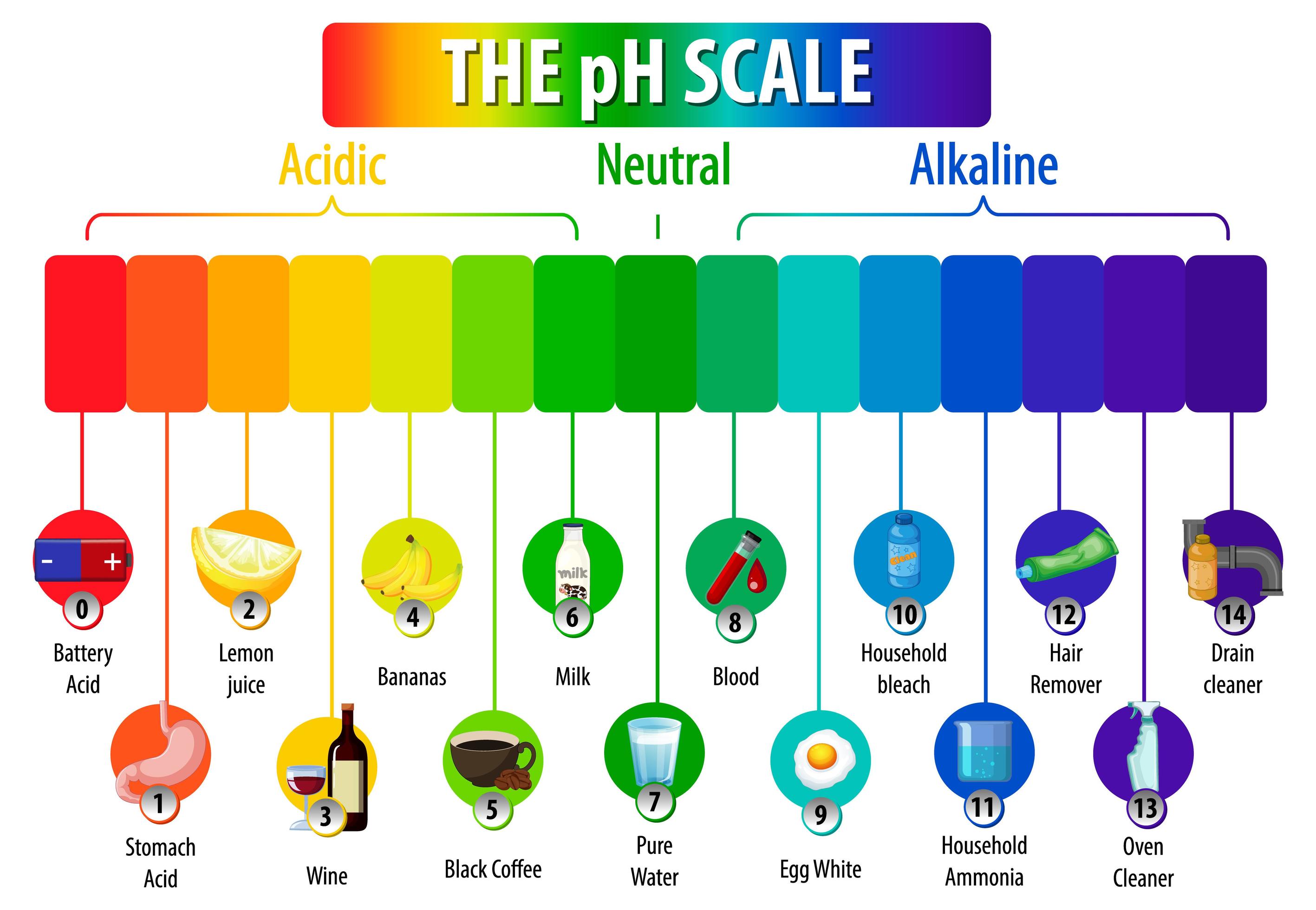
The pH Scale diagram on white background 1845080 Vector Art at Vecteezy
The pH scale ranges from 0 to 14. Let's see why it is up to 14 only and not more than 14. When acidic H3O+ and basic OH- combine, they form water. If we study the dissociation of water we can solve the mystery of 14. 2H2O ⇌ H3O+ + OH- Or H2O + H2O ⇌ H3O+ ++ OH-

Ph Scale Chart Vector Illustration Stock Illustration Download Image Now iStock
Detailed Description pH is a measure of how acidic/basic water is. The range goes from 0 - 14, with 7 being neutral. pHs of less than 7 indicate acidity, whereas a pH of greater than 7 indicates a base. pH is really a measure of the relative amount of free hydrogen and hydroxyl ions in the water.